In living organisms, individual cells move in complex 3D environments to establish organs, heal wounds, and hunt down pathogens. Yet much of what we know about cell movement has come from observing cells on 2D substrates, such as a glass coverslip. In 2D environments, cells generally move by extending a dynamic branched actin-filled leading edge. However, normal and cancer cells can use alternative forms of cell motility in 3D. These poorly described migration strategies are broadly called amoeboid migration. Our lab uses the beautiful developmental migration of Drosophila primordial germ cells (PGCs) as a model system to uncover the conserved molecular basis of amoeboid migration (See the movie below to get an idea of how stunning the process is).
PGCs are the founders of the germline, and their correct migration is essential for fertility. PGCs are a great model system because they invasively migrate through different tissues in a reproducible fashion and are amenable to high-resolution live imaging (our technique of choice, see the gallery) and genetic manipulation. Overall, our lab takes a multifaceted research approach- We use advanced microscopy and genetics to observe and perturb PGC migration in living embryos. We extract PGCs for biochemical assays and place them in bioengineered environments to test specific aspects of motility. Along the way, we build new tools to help us more precisely observe and manipulate PGCs. We welcome bioengineers, cell biologists, biochemists, biophysicists, and researchers from other fields to join us in our quest to understand how an amoeboid cell moves forward. Please continue below to see specific projects.
research Areas
Cytoskeleton
How does an amoeboid cell move? We found that PGCs curiously maintain a rounded shape during directed migration in vivo (movie (1)). This morphology is a hallmark of amoeboid migration, where cell movement is powered by flows of the actin cortex, called cortical flow. When these flows continuously travel from the front to the back of the cell, the cell travels forward, similar to how a tractor is propelled forward by its rear-moving tread. Alternatively, when cortical flows are not directed to a particular region, an amoeboid cell will spin in place. This occurs when PGCs have arrived at their embryonic target (movie (2)). Excitingly, we have found that purified PGCs (PGCs isolated from embryos) display constitutive cortical flows in the absence of any mechanochemical inputs (movie (3), compare it to movie (2)), mirroring their behavior in embryos. Thus, these cortical flows arise from the internal biochemistry of PGCs. We hypothesize there are actin-binding proteins and/or other novel factors enriched in PGCs that enable this. Moreover, we think the actin-binding proteins in PGCs create a particular cortical architecture that is conducive to flow, which we can visualize live when PGCs are placed under agarose (movie (4)).
(1) PGCs directionally migrating from endoderm to mesoderm. Black shows F-actin in PGCs in all movies.
(2) Cortical flows traveling around the periphery of PGCs, causing them to stay in place after arriving at their target in the mesoderm.
(3) Cortical flows traveling around the perimeter of an isolated PGC on a glass coverslip without any external mechanochemical inputs.
(4) Cortex architecture during flow can be visualized when PGCs are placed under agarose.
Traction forces
How are cortical flows in the interior of a cell transmitted to the external environment to propel an amoeboid cell forward? In the absence of any force coupling between cortical flow and the environment, a cell would remain fixed in place (movie (1)). We hypothesize that transmembrane proteins linked on the interior to actin are pulled rearward by cortical flows to generate traction forces for cell movement (movie (2)). We seek to identify what these ‘molecular paddles’ are. We are also asking if these paddles need to interact with specific molecules in the environment.
(1) Animated amoeboid cell with cortical flows (actin cortex shown in orange and yellow) traveling from front to back (red arrows).
(2) Animated amoeboid cell with cortical flows (actin cortex shown in orange and yellow) traveling from front to back (red arrows) that are coupled to ‘molecular paddles’, enabling movement.
Cell Signaling
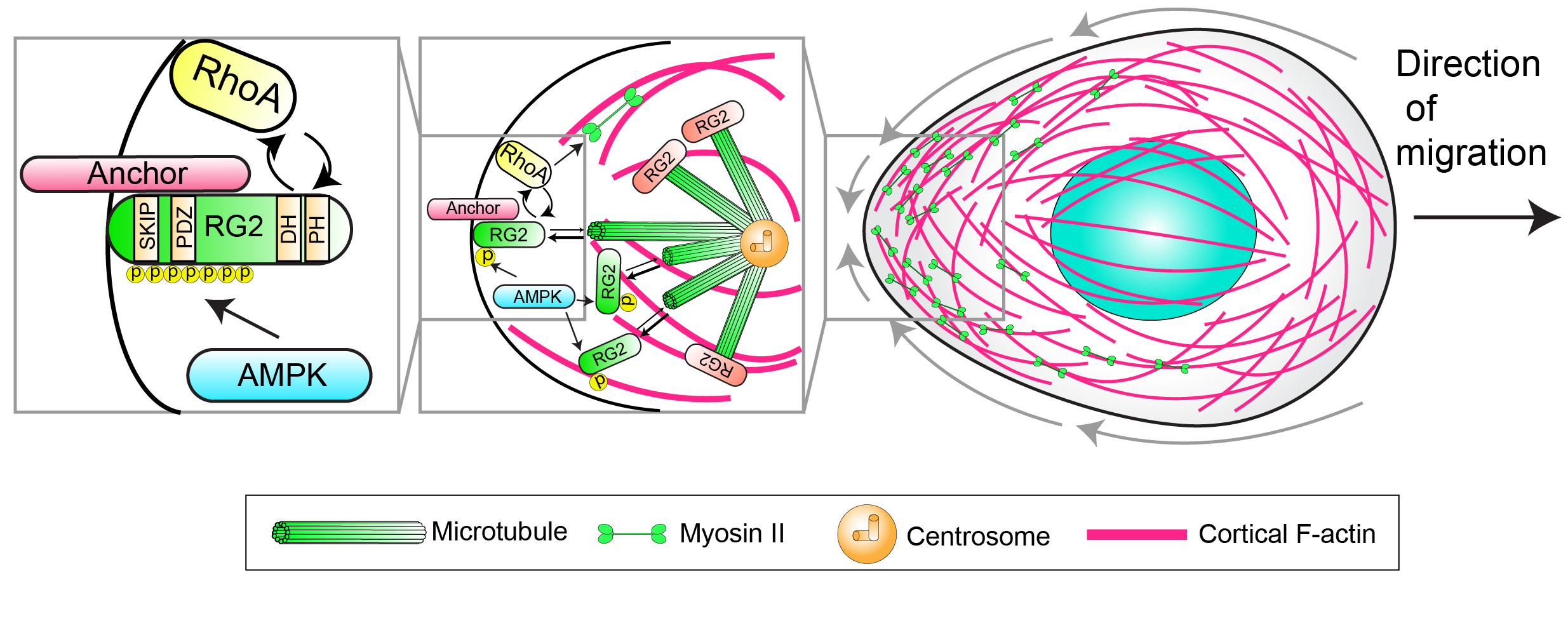
How does an amoeboid cell direct its movements to a specific location? We take advantage of the developmental migration of PGCs, which involves directed migration to stereotypic sites, to uncover navigation signaling pathways. PGCs use G-protein coupled receptors (GPCRs) to detect external attractants to direct their movements, but the downstream pathways are unclear. The key to guiding an amoeboid cell is controlling the direction of cortical flows. Seminal work in the C. elegans zygote has shown that cortical flows are drawn toward regions of high actomyosin contractility, where non-muscle myosin II motors contract cortical actin. Myosin II is enriched at the rear of PGCs, and we have delineated an upstream pathway that controls its activation at the back (see pathway below). We now seek to connect this rear pathway to the GPCR.
AMPK phosphorylates RhoGEF2 (RG2), locally liberating it from inhibitory microtubule interactions. RhoGEF2 then drives the activation of RhoA, which in turn activates myosin II at the rear.
Metabolism
Where does the energy to migrate come from, and how is the energy consumed by migration balanced with other cellular processes? We unexpectedly found that AMPK, a kinase typically associated with energy homeostasis, is involved in controlling the direction of PGC movement. AMPK is situated as a nexus between migration and energy sensing, and we seek to understand how it fulfills these roles. This is a new frontier for our lab, and we are excited to move into this field.